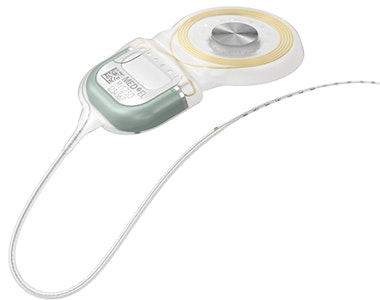
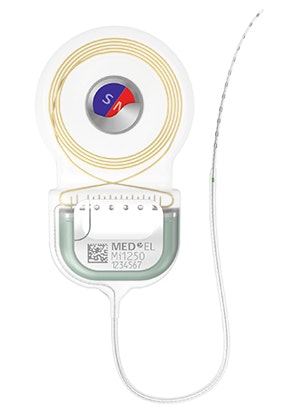
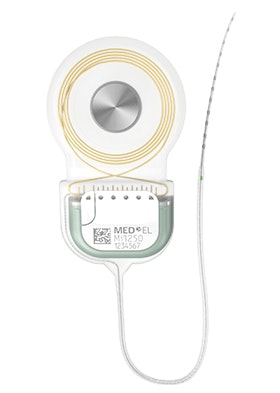
SYNCHRONY 2 Cochlear Implant
Made For You
SYNCHRONY 2 is our latest cochlear implant. SYNCHRONY 2 builds on the proven performance, MRI safety*, and security of SYNCHRONY. With a symmetrical electrode lead design, SYNCHRONY 2 delivers intuitive surgical handling with the smallest titanium cochlear implant.
- Closest to Natural Hearing
- Intuitive Surgical Handling
- 3.0 Tesla MRI Safety
Intuitive Surgical Handling
SYNCHRONY 2 has a symmetrical central electrode lead design for simplified implant placement. The streamlined electrode lead offers easier lead management and optimal surgical handling. Of course, the intracochlear electrode arrays are still MED-EL’s proven portfolio of full-length electrode arrays.
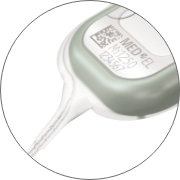
Central Electrode Lead
Symmetrical central electrode lead design for simplified surgical placement.

Green Marker Dot
Enables better visibility and control of electrode array insertion depth.

Optimal Lead Handling
Streamlined electrode lead offers easier lead management for optimal surgical handling.

PIN Housing
Titanium fixation pins easily secure the placement of the implant for long-term stability.


Awarded to the SYNCHRONY System, comprising the SONNET 2 Audio Processor and accessories, SYNCHRONY 2 Cochlear Implant, and MAESTRO 8.0 fitting software.

Closest to Natural Hearing
Above all else, the purpose of a cochlear implant is to create the most useful interface between the electrode array and the neural structures of cochlea. Every single element that goes into a cochlear implant culminates in this intricate bridge between technology and nature.
How do we provide the most natural hearing possible with a cochlear implant? By engineering our electrode arrays to most closely match the precise natural design and function of the cochlea.
Our incredibly flexible electrode arrays help better protect the delicate structures in the cochlea, enabling atraumatic insertion all the way to the apical region. This allows our full-length electrode arrays to provide two full turns of Complete Cochlear Coverage, which maximizes the natural tonotopic stimulation range. Finally, our unique variable-rate FineHearing sound coding is the only sound coding that mimics the temporal phase-locking in natural hearing for a sound quality that no other cochlear implant can match.
Structure Preservation
Our flexible electrode arrays are designed to protect natural structures and enable reliable scala tympani placement.
Complete Cochlear Coverage
Long, flexible arrays enable two full turns of stimulation for more accurate place-pitch match and more natural sound quality.
FineHearing Sound Coding
FineHearing technology combines rate coding and place coding to mimic natural sound coding for more natural sound quality.


Peace of Mind
We engineer all of our implants to deliver the long-term safety and reliability that your patients can always count on.
Every MED-EL implant is precision-built by expert technicians at our headquarters in Europe. We use state-of-the-art manufacturing facilities, cutting-edge processes, superior components, and exceptional quality control. This ensures every MED-EL cochlear implant can meet the highest standards of quality and reliability.
Independent Safety Capacitors
Our proven Ti100 CI electronics platform utilizes independent safety capacitors on every electrode channel for precise high-rate stimulation without any harmful DC current.
Proven Titanium Implants
As the 4th generation of our remarkably reliable titanium cochlear implant series, SYNCHRONY 2 is designed for many years of reliable performance.

Why MED-EL:
A Trusted Partner
For more than 30 years, MED-EL has been a trusted partner and innovation leader in hearing implants. We’re here to work together with you, and we’re committed to providing outstanding service and support for our professional partners.
With the most advanced cochlear implant systems, we offer the best hearing experience for your patients and the best clinical experience for you.
Contact Us
Ready to learn more about MED-EL cochlear implants?
Fill out our simple contact form and we’ll get in touch with you.
MAESTRO 11
With enhanced features and SONNET 3 support, MAESTRO 11 makes providing in-clinic and remote care to your patients with cochlear implants easy.

Electrodes
Find out what sets MED-EL electrode arrays apart from any other design to enable a closer to natural sound quality that no other cochlear implant can match.

SONNET 3
Smaller and lighter, SONNET 3 delivers built-in direct streaming and the closest to natural hearing with maximum flexibility and freedom.

RONDO 3
Find out how wireless charging, directional microphones, and wireless connectivity make RONDO 3 incredibly simple and simply incredible.
Technical Data


SYNCHRONY 2 Pin
Cochlear Implant (Mi1250)
Stimulation Features
- Sequential non-overlapping stimulation on 12 electrode channels
- Simultaneous (parallel) stimulation on 2 to 12 electrode channels
- 24 independent current sources
- Stimulation reference electrode on titanium housing
- Stimulation rates of up to 50,704 pulses per second
- Range of pulse phase duration: 2.1–425.0 μs/phase
- Time resolution (nominal values): 1.67 μs
- Current range (nominal value): 0–1200 μA per pulse phase
Pulse Shapes
- Biphasic, symmetric triphasic and triphasic precision pulses
Comprehensive Diagnostic Toolkit
- Status Telemetry
- Impedance and Field Telemetry (IFT)
- Electrophysiology measurements reference electrode on titanium housing
- Auditory Nerve Response Telemetry (ART™)
- Electrically Evoked Auditory Brainstem Response (EABR)
- Electrically Evoked Stapedius Reflex Threshold (ESRT)
- Electric Acoustic Evoked Potential (EAEP)
Housing Design
- Impact resistance up to 2.5 Joule
- Unique PIN variant with fixation pins for additional stability
- Hermetically sealed titanium housing
- Stimulator: 18.8 mm x 24 mm x 4.5 mm
- Coil: 29.0 mm diameter x 3.3 mm thick (typical)
- Weight: 7.7 g
Safety Features
- Independent safety capacitors for each electrode channel
- Unique Implant ID (IRIS)
- Biocompatibility standard according to ISO 10993-1
- Latex-free***
MRI Conditions****
- MR Conditional at 0.2, 1.0, 1.5 and 3.0 Tesla
- No magnet removal required even at 3.0 Tesla
Removable S-Vector Magnet
- Removeable S-Vector magnet for minimized image distortion
- Rotatable magnet within hermetic titanium housing
- Self-aligning to external magnetic field
- Conical shape for secure placement
Electrode Arrays
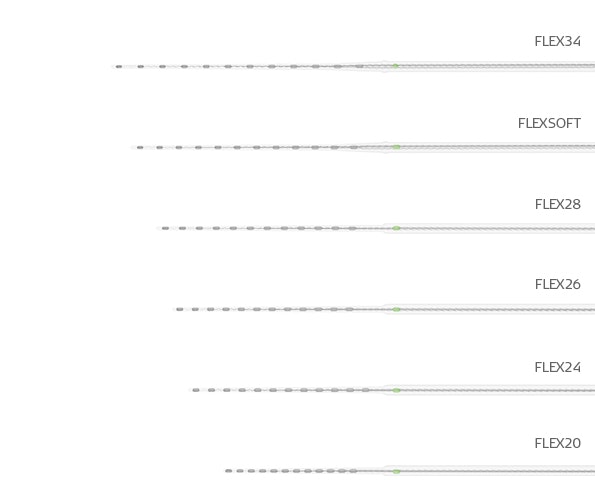
FLEX Series
The softest and most flexible electrode arrays, designed for Structure Preservation and Complete Cochlear Coverage. Featuring 19 active and physical platinum electrode contacts and FLEX-tip technology for atraumatic insertion. All FLEX series electrodes feature a green orientation marker for improved visibility and positioning during insertion.
FLEX34
- 28.6 mm stimulation range
- Diameter at basal end: 1.3 mm
- Dimensions at apical end: 0.5 x 0.4 mm
FLEXSOFT
- 26.4 mm stimulation range
- Diameter at basal end: 1.3 mm
- Dimensions at apical end: 0.5 x 0.4 mm
FLEX28
- 23.1 mm stimulation range
- Diameter at basal end: 0.8 mm
- Dimensions at apical end: 0.5 x 0.4 mm
FLEX26
- 20.9 mm stimulation range
- Diameter at basal end: 0.8 mm
- Dimensions at apical end: 0.5 x 0.3 mm
FLEX24
- 20.9 mm stimulation range
- Diameter at basal end: 0.8 mm
- Dimensions at apical end: 0.5 x 0.3 mm
FLEX20
- 15.4 mm stimulation range
- Diameter at basal end: 0.8 mm
- Dimensions at apical end: 0.5 x 0.3 mm
FORM Series
Designed specifically for malformed cochleae and for instances where leakage of cerebrospinal fluid (CSF) is expected. Featuring 24 active and physical platinum electrode contacts and SEAL technology designed to aid closing of the cochlear opening.
FORM24
- 18.7 mm stimulation range
- Diameter at basal end: 0.8 mm
- Diameter at apical end: 0.5 mm
FORM19
- 14.3 mm stimulation range
- Diameter at basal end: 0.8 mm
- Diameter at apical end: 0.5 mm
CLASSIC Series
Features 24 active and physical platinum electrode contacts.
STANDARD
- 26.4 mm stimulation range
- Diameter at basal end: 1.3 mm
- Diameter at apical end: 0.5 mm
MEDIUM
- 20.9 mm stimulation range
- Diameter at basal end: 0.8 mm
- Diameter at apical end: 0.5 mm
COMPRESSED
- 12.1 mm stimulation range
- Diameter at basal end: 0.7 mm
- Diameter at apical end: 0.5 mm
* The SYNCHRONY 2 cochlear implant is MR conditional. Recipients with a SYNCHRONY 2 cochlear implant may be safely MRI scanned at 1.5 and 3.0 Tesla following the conditions detailed in the instructions for use.
** Unless required for diagnostic reasons.
*** Whereby “free” means “not made with latex” according to current FDA guidance.
**** It has been demonstrated that no known hazards exist in specified MRI environments under conditions as described in the SYNCHRONY 2 cochlear implant product labeling. The SYNCHRONY 2 cochlear implant is MR conditional. Recipients with a
SYNCHRONY 2 cochlear implant may be safely MRI scanned at 0.2, 1.0, 1.5, and 3.0 Tesla following the conditions detailed in the instructions for use.
- O’Connell, B.P., Cakir, A., Hunter, J.B., Francis, D.O., Noble, J.H., Labadie, R.F., Zuniga, G., Dawant, B.M., Rivas, A., & Wanna, G.B. (2016). Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol Neurotol. 37(8):1016–1023.
- O’Connell, B.P., Hunter, J.B., Haynes, D.S., Holder, J.T., Dedmon, M.M., Noble, J.H., Dawant, B.M., Wanna, G.B. (2017). Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope. 127(10):2352-2357
- Analysis based on mean cochlear values and clinical data. Data on file.
- Rader, T., Döge, J., Adel, Y., Weissgerber, T., & Baumann, U. (2016). Place dependent stimulation rates improve pitch perception in cochlear implantees with single-sided deafness. Hear Res., 339, 94–103.
- Müller, J., Brill, S., Hagen, R., Moeltner, A., Brockmeier, S.J., Stark, T., Helbig, S., Maurer, J., Zahnert, T., Zierhofer, C., Nopp, P., & Anderson, I. (2012). Clinical trial results with the MED-EL fine structure processing coding strategy in experienced cochlear implant users.
- Killan, C., Scally, A., Killan, E., Totten, C., & Raine, C. (2019). Factors affecting sound-source localization in children with simultaneous or sequential bilateral cochlear implants.