BONEBRIDGE BCI 602
Active Bone Conduction Implant

Always a Step Ahead
By combining powerful direct-drive amplification with proven transcutaneous technology in a streamlined 2nd generation implant design, the BONEBRIDGE BCI 602 offers an unmatched level of hearing performance and surgical flexibility[ft][ft].

Awarded 2019 to the BONEBRIDGE BCI 602 Bone Conduction Implant.
- Direct-Drive Active Bone Conduction
- Straightforward Surgery
- Long-Term Reliability

Hearing Performance
Bone conduction systems can be an ideal solution for mixed or conductive hearing loss, but not all bone conduction implants are created equal. Your patients are choosing to hear—why not choose to hear their best?
BONEBRIDGE delivers our proven combination of direct-drive active amplification and wireless transcutaneous signal transmission for excellent hearing outcomes across a wide range of candidacies.
- Direct-Drive
- Powerful Amplification
- Feedback-Free

Healthy Skin
Why is healthy, happy skin so important for every recipient? An audio processor only works when it’s worn—and patients deserve reliable, complication-free hearing. That’s why we designed BONEBRIDGE and SAMBA 2 for all-day comfort in any environment.[ft][ft]
- Intact Skin
- No Pressure
- All-Day Comfort

Straightforward Surgery
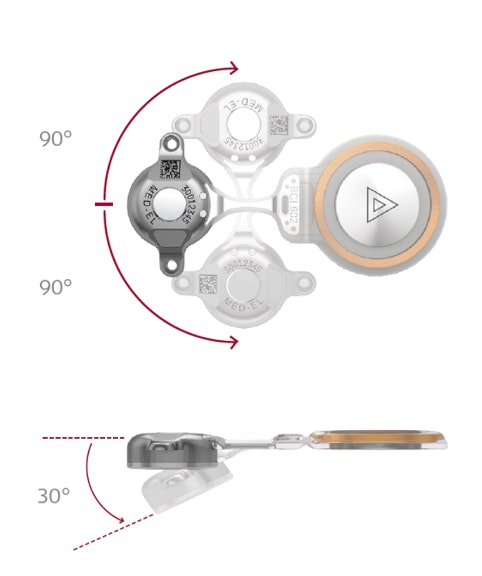
Through close collaboration with surgeons and clinicians, the 2nd generation BONEBRIDGE BCI 602 was engineered from the ground up to deliver optimal surgical handling and reliable implant fixation. With nearly 50% less drilling depth, solid 2-point fixation, self-drilling screws, and flexible implant positioning, the BCI 602 delivers exactly what you’re looking for.
- Flexible Placement
- Easy Fixation
- ~50% less drilling depth

Peace of Mind
With a robust implant design and secure titanium fixation, BONEBRIDGE offers years of reliable hearing performance. No other bone conduction implant can match our record of long-term safety and reliability. With BONEBRIDGE, you’ll have peace of mind knowing your patients are in good hands.[ft]
360° Reliability by Design
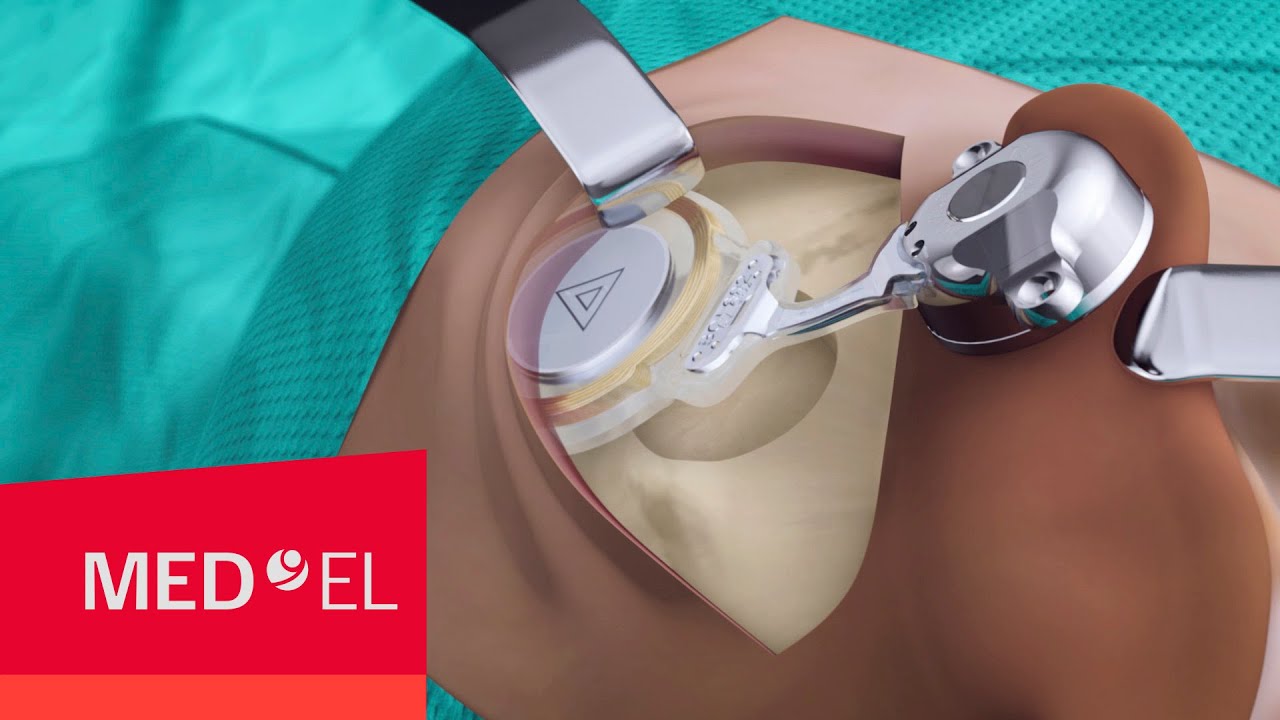
With a bone conduction implant, a reliable connection to the bone is essential. However, traditional BAHA-style implant screws have been shown to be vulnerable to osseointegration failure. Even newer single-point bone anchored systems may be at risk from external impact.
BONEBRIDGE is a robust implant engineered to minimize complications, withstand impact, and keep skin healthy. Using our proven two-point fixation with the titanium transducer resting solidly against the bone, BONEBRIDGE provides safe and reliable hearing by design.[ft]
- Transducer recessed securely in the bone
- Lower complication rate
- Over a decade of unmatched, long-term clinical experience

Why MED-EL:
A Trusted Partner
For more than 30 years, MED-EL has been a trusted partner and innovation leader in hearing implants. We’re here to work together with you, and we’re committed to providing outstanding service and support for our professional partners.
With the most advanced hearing implant systems, we offer the best hearing experience for your patients and the best clinical experience for you.
Contact Us
Ready to learn more about BONEBRIDGE?
Fill out our simple contact form and we’ll get in touch with you.
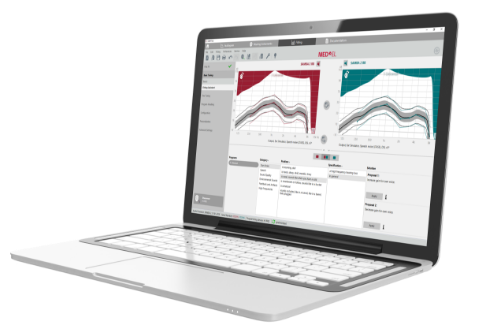
SYMFIT 8.0
The SYMFIT 8.0 software makes fitting SAMBA 2 easy and efficient. Its straightforward guided workflow includes precise Vibrograms and fitting tools to help you get the most out of SAMBA 2.

SAMBA 2
SAMBA 2 for VIBRANT SOUNDBRIDGE and BONEBRIDGE combines intelligent hearing technology with easy handling, intuitive connectivity options and improved fitting software to make hearing as simple as possible—for both hearing implant recipients and their audiologists.
Technical Data
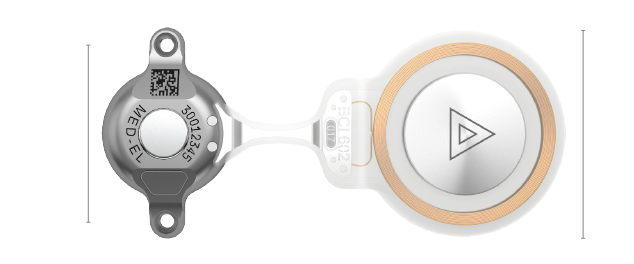
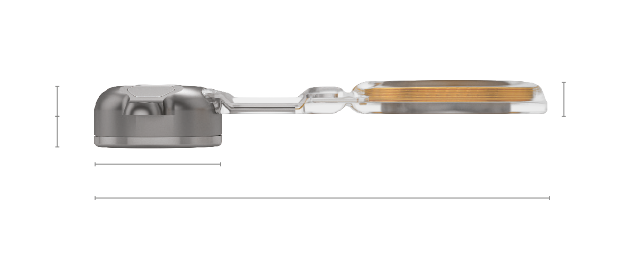
BCI 602 Implant
Implant Kit Contents
- 1 BCI 602 Bone Conduction Implant
- 3 Cortical Screws (Osseointegration of the screws is not required):
- 2 Self-Drilling Standard Screws 1.6 x 5 mm (silver)
- 1 Emergency Screw 1.9 x 5 mm (blue)
- 1 Surgical Screwdriver SD 2 (single-use)
Weight
Approx. 20 g
Materials in Contact With Tissue
Implant: Medical grade silicone elastomer, titanium grade 5 ELI (in accordance with ASTM F 136-12)
Screws: Titanium alloy Ti6Al7Nb
Screwdriver
Polyoxymethylene (POM), martensitic stainless steel (1.4197)
MRI Conditions
MR-conditional at 1.5 Tesla*
Maximum Diving Depth
50 m in salt water (6 bar)
Biocompatible according to ISO 10993-1
Latex-free***
Delivered sterile
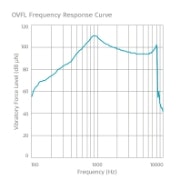
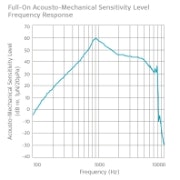
Frequency Response Curves
SAMBA 2 BB
Maximum OVFL90: 110 dB VFL
HFA-OVFL90: 102 dB VFL
Maximum full-on acousto-mechanical sensitivity level: 59 dB AMSL
Full-on HFA-AMSL: 50 dB AMSL
Equivalent input noise: 21 dB SPL
Total harmonic distortion, 70 dB SPL input level,
500 Hz distortion test frequency: < 5 %
Total harmonic distortion, 70 dB SPL input level, 800 Hz distortion test frequency: < 2 %
Total harmonic distortion, 65 dB SPL input level, 1600 Hz distortion test frequency: < 5 %
Total
harmonic distortion, 60 dB SPL input level, 3200 Hz distortion test frequency: < 2 %
* The BONEBRIDGE bone conduction implant is MR conditional. Recipients with BONEBRIDGE may be safely MRI scanned at 1.5 Tesla following the conditions detailed in the instructions for use.
*** Whereby “free” means “not made with latex” according to current FDA guidance.