360° View of Bone Conduction
Implant Safety and Reliability

Our Philosophy
All our life-changing devices are developed with a complete 360-degree view of reliability and safety in mind. BONEBRIDGE, MED-EL’s active bone conduction implant system, is the most advanced active transcutaneous bone conduction system available on the market—and it’s no coincidence that BONEBRIDGE provides outstanding reliability.
We designed BONEBRIDGE with a broad understanding of what is needed to make a bone conduction implant system safe and reliable: a robust implant engineered to minimize complications, withstand impact, keep skin healthy, and allow easy access to MRI scans.* And all that comes with a reliable audio processor that makes everyday hearing simple for recipients.
We know your patients count on our technology to enjoy life every day. That’s why we made BONEBRIDGE safe and reliable by design.
BONEBRIDGE: Over a Decade of Clinical Data
The second generation BONEBRIDGE, BCI 602, was engineered in close collaboration with surgeons and clinicians to further optimize the design of the world’s first active transcutaneous bone conduction implant. Now, with over a decade of clinical experience and thousands of implantations worldwide, BONEBRIDGE has a proven track record of outstanding reliability.
In fact, over a decade of studies show BONEBRIDGE has a lower rate of complications and adverse events than both percutaneous and passive transcutaneous bone conduction implants.[ft][ft][ft][ft][ft][ft][ft][ft][ft]
- 360° reliability by design
- Lower complication rate
- Over a decade of unmatched, long-term clinical experience

Clinical Safety
While it is important for implant manufacturers to report reliability data openly, these reports only provide part of the picture. Clinic-reported data in peer-reviewed publications often give a more complete overview. These are especially useful when it comes to complications and adverse events associated with a device.

Impact Safety
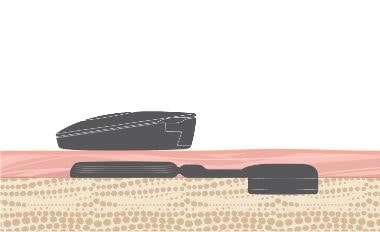
We engineered BONEBRIDGE for impact resistance. A robust titanium design, stable two-point fixation, and a transducer embedded in the bone with low protrusion make BONEBRIDGE very reliable against impact.
- Stable two-point fixation
- Transducer recessed securely in bone
- Lower protrusion above the bone[ft]

Skin Safety
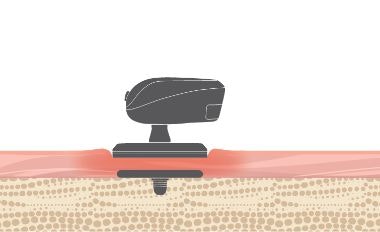
BONEBRIDGE is designed to leave the skin over the implant intact. The implant is placed beneath the skin and the transducer transfers vibrations directly to the bone. This allows the skin to remain healthy.
- Intact Skin
- No permanent wounds
- No pressure

Made for MRI
BONEBRIDGE’s advanced magnet technology enables its magnet to remain secure and free of force throughout MRI scans. This enables straightforward and easy access to 1.5T MRI scans following the simple safety conditions—all without additional surgery and without hearing downtime.*
Great Protection. Guaranteed.
Because of our long and positive experience with MRIs and hearing implants, we offer a life-long MRI guarantee. We promise to replace a BONEBRIDGE implant in the very unlikely event that it’s damaged during an MRI scan.
This life-long MRI guarantee for hearing implants is the first and only such guarantee to be offered by any hearing implant manufacturer. It covers both the BCI 601 and BCI 602 implants, so your patients can feel confident about any MRI scans they need in the future.
- Hassle-free access to 1.5T MRI scans*
- No surgery
- No hearing downtime

Audio Processor Reliability
An implant that delivers outstanding reliability would be no good without an audio processor that can also do the same. An audio processor that is reliable by design provides your patients with reliable hearing day after day. This also means there will be fewer visits to your clinic.
Low Average Monthly Repair Rate
Our audio processors—SAMBA and SAMBA 2—have a proven record of outstanding reliability. And that’s good news because with fewer issues, you’ll save time on fitting replacements and administrating returns.

Active Bone Conduction Implants: Safety & Reliability Comparison
Your patients deserve devices that are safe and reliable by design. When choosing between the two active bone conduction implant systems available on the market, there are several important factors to keep in mind.
| BONEBRIDGE | Osia** | |
| Minimal skin strain thanks to ergonomic, rounded design [ft][ft] | ||
| Implant transducer recessed in bone bed provides stability with a low profile [ft][ft] | ||
| Proven electromagnetic transducer design for over a decade | ||
| Secure, two-point fixation provides additional stability [ft][ft] | ||
| Impact-tested according to ISO 14708-7:2019: implant can withstand impact up to 2.5 joules [ft][ft] | ||
| Unmatched, long-term clinical experience for over a decade [ft] |

MED-EL:
360° Reliability by Design
For more than 35 years, MED-EL has been a trusted partner and innovation leader in hearing implants. We create and manufacture our implants directly in our Austrian headquarters, ensuring they follow state-of-the-art European engineering, and meet the highest standards in quality and reliability. With this focused oversight and our dedication to quality, we can check and verify every single step of the design and process.
MED-EL has always been privately owned by our founders, so for us reliability is not a percentage or an earnings report for investors. With no shareholders to answer to, we’re in a unique position where we can put the safety and peace of mind of our recipients above all else. And because we believe that everyone should benefit from the latest hearing technology, our latest audio processors work for all our recipients since 1994.
Choosing MED-EL means putting your trust in us. We promise to always be there for you, our professional partner, and for your patients.
- European engineering
- Manufactured on-site
- Dedication to reliability and quality
- Unmatched backward compatibility
- Committed to recipients
Contact Us
What to learn more about how BONEBRIDGE is reliable by design? Fill out the form below and a MED-EL representative will get in touch with you.
* The BONEBRIDGE BCI 601 and BCI 602 Active Bone Conduction Implants are MR Conditional. Recipients with BONEBRIDGE may be safely MRI scanned at 1.5 Tesla following the conditions detailed in the instructions for use.
** Osia is a trademark of Cochlear Ltd, Australia
.jpg?auto=format&sfvrsn=ddc38e43_0)